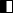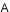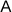Stem Cell Differentiation and Cytogenetics Group
Group leader: Dr. Insa S. Schröder
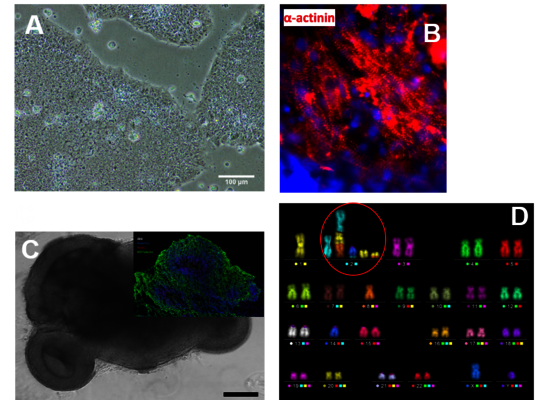
At the Stem Cell Differentiation and Cytogenetics Group, we are interested in the effects of ionizing radiation on organs such as the brain, the heart, and the lung trying to elucidate the risks for patients treated with ionizing radiation as well as for astronauts, who are inevitably exposed to ionizing radiation on their missions into space. For this purpose, we use human stem cells that play a pivotal role in tissue development, maintenance and function. Particularly, human embryonic stem cells (hES cells), which can give rise to all tissues of an organism (Figure A), are used to generate mature cells of the heart (Figure B) allowing us to study radiation/microgravity-induced cardiovascular alterations, which may occur during long-term space missions. hES cells also serve as a basis for cerebral organoid models (Figure C) to better understand e.g. the mechanisms of cognitive impairment and radiation necrosis as severe side effects of radiation therapies. In such organoids, we can also mimic brain tumor initiation and growth and examine the interaction between normal tissue and tumor tissue upon treatment with ionizing radiation alone or in combination with other treatment modalities such as chemotherapies. Finally, using primary lung stem cells or lymphocytes from different donors, we study the effects of radon on patients, who are treated in radon spas or baths to alleviate rheumatic diseases. All research areas are substantiated by analyses (Figure D) monitoring the acute radiation-induced cytogenetic damage and the long-term genetic stability of cells.
(A) Colonies of human embryonic stem cells, bright field microscopy, scale bar: 100µm. (B) Human cardiomyocytes immunostained for α-Actinin (red). (C) Cerebral organoid, scale bar 1000µm, insert: organoid section immunostained for Pax6 (red) and ßIII-Tubulin (green), nuclei counterstained with Hoechst 33342 (blue). (D) Karyotype of a human cell after heavy ion exposure revealed by mFISH technique. The cell harbors a complex aberration involving several chromosomes (red circle).
Main research topics
- Radiation response of human brain, heart, and lung tissue
- Differentiation/regeneration
- Functionality
- Genetic stability
- Response of brain tumor cells and their interaction with normal brain cells after
radiation therapy or combinatorial chemotherapy/radiation therapy
- Radiation-induced cytogenetic damage and genetic stability in human peripheral
blood lymphocytes- Effects of radon spa therapy (in vivo studies)
- Effects of radon spa therapy (in vivo studies)
- Dose-response relationships for different radiation types (neutrons, radon, alpha
particles, HZE-particles, X-rays) over a wide range of LETs/energies (in vitro studies).
Collaborations
- University of Applied Sciences Aschaffenburg, Germany (Professor C. Thielemann)
- Joint Institute for Nuclear Research, Dubna, Russia (Dr. E. Nasonova)
- Technical University of Ilmenau, Germany (Prof. A. Schober)
- German Aerospace Center, Institute of Aerospace Medicine, Gravitational Biology
(PD R. Hemmersbach,Dr. C Liemersdorf) - Federal Office for Radiation Protection, Neuherberg, Germany (PD Dr. O. Azimzadeh)
- Technical University of Darmstadt, Germany (Prof. B. Laube, Prof. M. Löbrich, Prof. G. Thiel)
- Heidelberg University Hospital, Germany (Prof. J. Debus)
- Martin-Luther-Universität Halle-Wittenberg, Institut für Physiologische Chemie (IPC)
- AG Stammzellbiologie, Germany (Dr. M. Jung)
- Psychotherapie und Psychosomatik, Germany (Dr. M. Jung)
- MD Anderson Cancer Center, University of Texas, Houston, Texas, USA (Prof. D. Grosshans)
Funding
- NIH 1R01CA256848- 01 “Determining the optimal ion and fractionation scheme
for the treatment of GBM in a comprehensive human organoid model” - BMBF 02NUK049A (Verbundprojekt Brain Radiation Assay: Etablierung eines in vitro
Systems zur Analyse und Prädiktion von Schäden im zentralen Nervensystem nach
Expositionmit ionisierender Strahlung in Kombination mit anderen Neurotoxika) - BMBF 02NUK050A (Verbundprojekt GREWIS-alpha: Genetische Risiken und
entzündungshemmende Wirkung von dicht-ionisierender alpha-Strahlung) - HGS-HIRE (Helmholtz Graduate School for Hadron and Ion Research)
Group members
- Research scientists:
Dr. Tamara Bender (Postdoc)
- Technicians / Laboratory Engineers:
Leonie Hartig
Emilia Choman
Dr. Carola Hartel (L. E.)
- PhD students:
Esther Schickel
Kim Knorr
Leon Kaysan
Selected publications
- Schroeder IS, Pluripotent Stem Cells for Cell Therapy, In Vitro Models for Stem Cell Therapy, Methods in Molecular Biology, 25 (2021), ISBN: 978-1-0716-1224-8, DOI: https://doi.org/10.1007/978-1-0716-1225-5
- Schielke et al., Solving the issue of ionizing radiation induced neurotoxicity by using novel cell models and state of the art accelerator facilities. Front. Phys. 2020 Sep; 8:568027.doi: 10.3389/fphy.2020.568027
- Mayer M et al., Novel in vitro assay to investigate radiation induced changes in the functionality of human embryonic stem cell-derived neurospheres. Neurotoxicology. 2020 Jul; 79:40-47. doi: 10.1016/j.neuro.2020.04.003. Epub 2020 Apr 19.
- Hartel C et al., Persistence of radiation-induced aberrations in patients after radiotherapy with C-ions and IMRT. Clin Transl Radiat Oncol. 13, 57 (2018). doi: 10.1016/j.ctro.2018.10.002. eCollection 2018 Nov. PMID: 30364751 Free PMC article.
- Nitsch S et al., Functional video-based analysis of 3D cardiac structures generated from human embryonic stem cells. Stem Cell Res.29, 115 (2018)
- Luft S et al., Ionizing radiation alters human embryonic stem cell properties and differentiation capacity by diminishing the expression of activin receptors. Stem Cells Dev. 26, 341 (2017)
- Helm A et al., Ionizing radiation impacts on cardiac differentiation of mouse embryonic stem cells. Stem Cells Dev. 25, 178 (2016)
- Luft S et al., Fate of D3 mouse embryonic stem cells exposed to X-rays or carbon ions. Mutat Res Genet Toxicol Environ Mutagen760, 56 (2014)
- Lee R et al., Chromosome aberration measurements in mitotic and G2-PCC lymphocytes at the standard sampling time of 48 h underestimate the effectiveness of high-LET particles. Radiat Environ Biophys 50, 371 (2011)
- Ritter S and Durante M, Heavy-ion induced chromosomal aberrations: a review. Mutat Res14, 701, 38 (2010)
- Hartel C et al., Chromosomal aberrations in peripheral blood lymphocytes of prostate cancer patients treated with IMRT and carbon ions. Radiother Oncol.95, 73 (2010)