In LD-RT (low dose radiotherapy), photons or densely ionizing radiation, e.g. in radon spas, is used to treat rheumatoid arthritis, ankolysing spondylitis, musculoskeletal disorders, psoriasis and others (Figure 1). Cellular and molecular mechanisms underlying the observed alleviation from pain in radon spa are widely unknown.
GREWIS / GREWIS-alpha Consortium
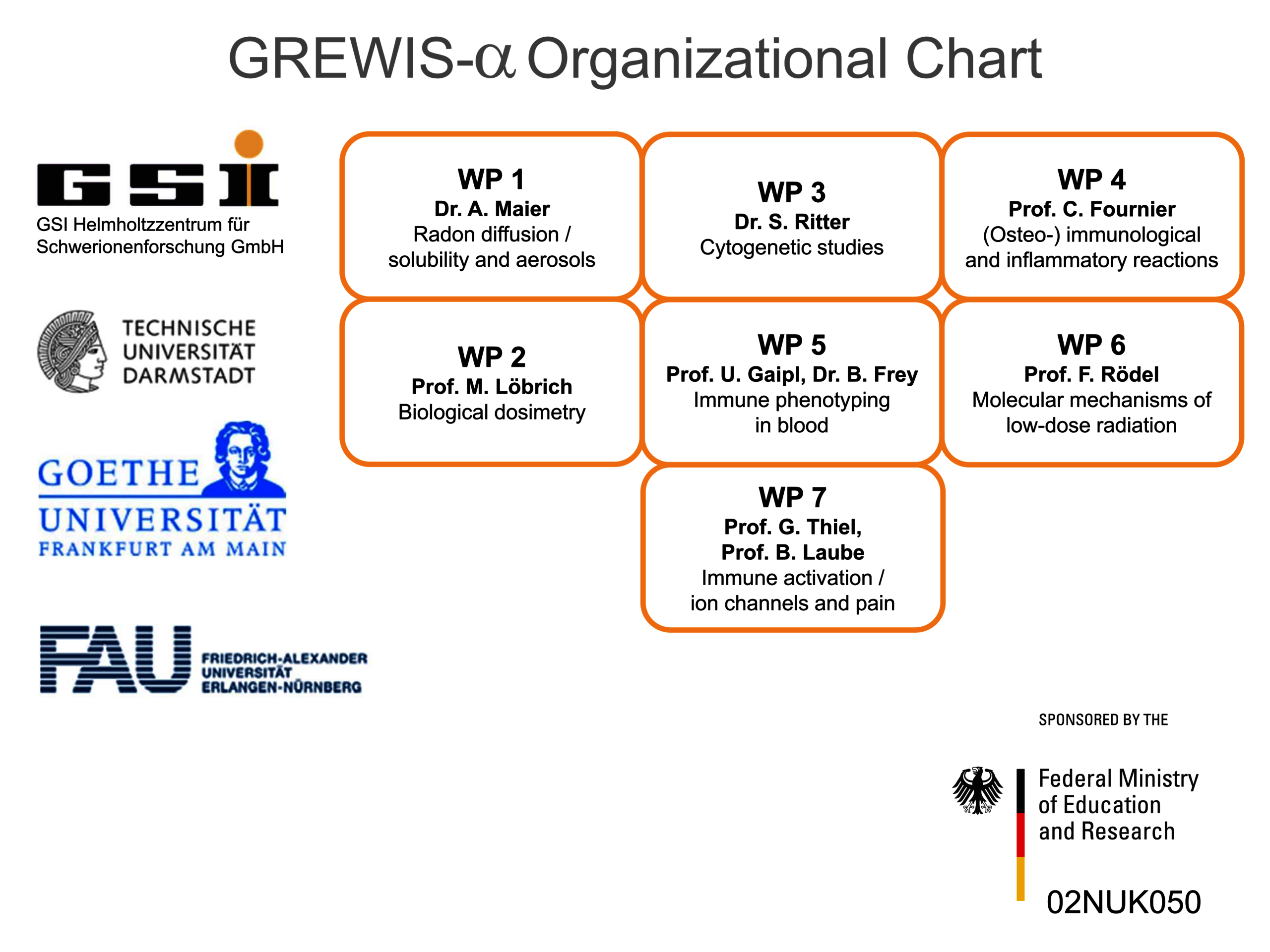
Therefore, we set out to investigate potentially beneficial and harmful aspects of low dose radon exposure. To achieve this, we work together in the frame of the „GREWIS“-project with experts covering the fields of particle physics, cytogenetics and DNA repair, immunology, molecular cell biology and physiology, s. Helmholtz Association. The GREWIS-alpha consortium includes 7 working groups from 4 institutions and associated medical collaborators (Figure 2).

Prof. Dr. Claudia Fournier, Dr. Andreas Maier, Dr. Denise Eckert, Dr. Ayele Taddese Tsedeke
The research groups of the GREWIS projects have established an integrated strategy to study several aspects of risk and the potential immune-modulating effect of low radiation doses.
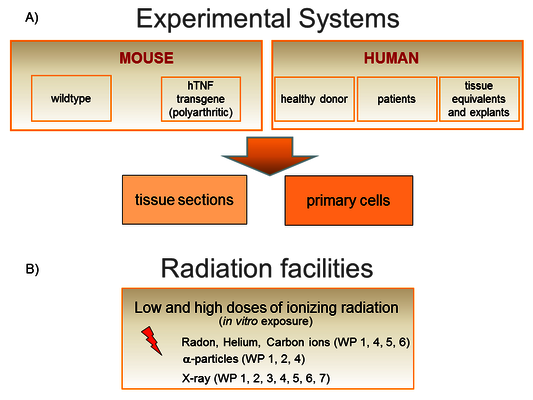
We work on a molecular and cellular level as well as in tissues and animals (Figure 3A). We have access to human bone marrow (hematopoietic and mesenchymal stem cells), skin from healthy donors, human knee tissue samples (infrapatellar fat pads and synovial membrane) and blood from patients undergoing radon therapy (RAD ‐ ON02, EudraCT 2016‐002085‐31) and low dose X-ray therapy (IMMO-LDRT; NCT02653079).
Furthermore, we have developed a unique tool for radon exposure: A chamber allowing radon exposure of cells and mice, operating under controlled conditions including humidity, temperature and radon concentration, which can be adjusted comparable to radon therapy or higher (Figure 4, click to enlarge) [1]. Diffusion and solubility measurements of radon and an analysis algorithm were developed [2]. Furthermore, using molecular dynamics simulations, an uneven distribution of radon between fatty acids and isotonic saline solution could be explained mechanistically [3].
In addition, an α-particle irradiation setup has been constructed and is available at GSI and TU Darmstadt. To this end, an Americium-241 source with comparable energies to the α-particles emitted by radon is used. With this source, cells can be irradiated on thin foils for defined time intervals [4].
Furthermore, heavy ions with similar characteristics as α-particles (helium and carbon ions) are used (Figure 3B). In order to be able to estimate the dose and thus also the risk of radon exposure, measurements were carried out on a volunteer patient after therapy in a radon gallery. The evaluation models developed here can be applied to other applications, for example for dose estimation in a mouse model or in physical samples.
Biodosimetry based on DNA damage markers after radon exposure has been established for different organs, and X-ray reference curves have been measured.
As blood vessels as well as the interaction of endothelial cells of the blood vessel wall and hematopoietic cells have a crucial role in the first steps of immune reactions, a “flow chamber” was constructed to mimic the shear stress caused by the blood flow.
Experiments are performed in vitro using both primary mouse and human cells (hematopoietic, skin, bone, synovial and fat cells; differentiated from stem and progenitor cells from healthy donors), also as 3D tissue equivalents.
An in vivo approach takes advantage of the hTNFα-transgenic mouse model and the K/BxN serum transfer model for polyarthritis to investigate maturation and activity of immune and bone cells.
Our first RAD-ON01 study revealed that patients have reduced pain after radon treatment. This could be due to a decrease in the number and activity of bone-resorbing osteoclasts and has also been confirmed in the patient study on photon radiation (IMMO-LDRT). Additionally, in the plasma of radon and LDRT patients, a decrease in collagen fragments and a lower activity of an enzyme involved in bone resorption (TRAP) could be detected. In a second ongoing RAD-ON02 study, a cross-over design was used for the first time, i.e. there is a control group and a group of radon-exposed patients for two consecutive years with the same 100 patients. With this design, a possible placebo effect should be eliminated. After a year, the patients are treated again, but the groups are swapped. The study is blinded, i.e. it is not known neither to patients, doctors nor to researchers which group received which treatment.
This is consistent with functional changes observed for low dose photon exposure (LDRT) in the polyarthritis mouse model and results obtained in vitro on the molecular level (Figure 5, click to enlarge) [5-14].
The current state of knowledge on the possible effect and risk of radon therapy was summarized and published in a review [15].
References:
[1] Maier et al., Nucl. Instr. Meth. Phys. Res. B, 362:187-193 (2015)
[2] Maier et al., Nucl. Instr. Meth. Phys. Res. B, 416:119-127 (2018)
[3] Sanjon et al., Scientific Reports 9,10768 (2019)
[4] Maier et al., Int. J. Radiat. Biol, 96:2, 206-213 (2020)
[5] Large et al., Rad. Oncology, 9:80 (2014)
[6] Large et al., Strahlenther Onkol, 191:742-749 (2015)
[7] Thangaraj et al., Chemico-Biological Interactions, 259B: 49-412 (2016)
[8] Roth et al., Pflügers Archive-European. J. Physiol. 467:1835-1849 (2014)
[9] Gibhardt et al., Scientific reports, 5:13861 (2015)
[10] Rühle et al., Autoimmunity, 50(2):133-140 (2017)
[11] Erbeldinger et al., Front Immunol., 8:627 (2017)
[12] Cucu et al., Front Immunol., 8:882 (2017)
[13] Maier et al., Nucl. Instr. Meth. Phys. Res. B, 416:119-127 (2018)
[14] Rühle et al., Modern Rheumatology, 29(1):165-172, (2019)
[15] Maier et al., Int. J. Mol. Sci. 22(1), 316 (2021)
[28] Shreder et al., Int. J. Mol. Sci.19(9): 2717 (2018)
[29] Rapp et al., Sci Rep 9(1):5000 (2019)