Clinical Radiobiology
Group leader: Dr. Walter Tinganelli
Walter holds a Master of Science in Biotechnology from the University of Naples Federico II and a Ph.D. in Radiobiology from the Technical University of Darmstadt in Germany. After completing his Ph.D., he served as the International Open Laboratory Group Director at Japan's National Institute of Radiological Sciences (NIRS) for two years. He then spent three years as a Principal Investigator at the Trento Institute for Fundamental Physics and Application, part of the National Institute of Nuclear Physics (INFN) in Trento, Italy. While in Trento, he set up the first laboratory and experimental room in the proton therapy center.
Since 2019, Walter has been the Clinical Radiobiology Group Leader at the Helmholtz Research Center for Heavy Ion Research (GSI) in Darmstadt, Germany.
Walter actively participates in various international organizations. He is a member of the Life Science Working Group of the European Space Agency (ESA), serves as the Chair of Biology (Europe) at the Particle Therapy Co-Operative Group (PTCOG), takes part in the FLASH Focus Group at the European Society for Radiotherapy & Oncology (ESTRO), and is a member of the steering committee of the Italian Radiation Research Society (SIRR).
- Hibernation as protection for space mission and radiotherapy
Hibernation has been proposed long ago as a tool for human space travel. Humans are non-hibernating mammals and for this reason, the research slowly stopped after the first human space missions. However, in recent years, a procedure to induce a metabolic state known as “synthetic torpor", has been developed.
Synthetic torpor may be not only an efficient method to spare resources and reduce psychological problems in long-term exploratory-class missions, but may also represent a key countermeasure against health risks caused by exposure to cosmic rays.
Moreover, hibernation, used in conjunction with radiotherapy could be the key to fighting cancer in the future. Hibernation-like 'deep sleep' state could hypothetically slow down their bodily functions and halt the spread of tumors inside the tissues, while also increasing the body's resistance to radiation. In this project, we study the effects of radiation on synthetic hibernation in collaboration with the group of Dr. Cerri (University of Bologna) and other international institutes and we deal with verifying with in vitro experiments the molecular mechanisms involved.
- Tumor Hypoxia
In clinical radiotherapy, hypoxia is a characteristic feature of locally advanced solid tumors. Those hypoxic tumor cells are very radioresistant and often responsible for local recurrences and a source of cancer stem cells, disseminating tumor cells and metastases, resulting in a poor prognosis. Reduced radiosensitivity in hypoxia was shown as far back as 1921. The sensitizing effect of oxygen can only be observed when oxygen is present at the time of irradiation, and the effect is dependent on the oxygen concentration. At oxygen concentrations > 3% the full sensitizing effect is observed, while at lower concentrations, especially from 1% to 0.1%, a steep decrease in radiosensitivity is measured (Oxygen Enhancement Ratio OER). High-LET radiation tumor therapy, such as carbon-ion therapy, offers the possibility of reducing the OER and inactivating hypoxic tumor cells more efficiently.
In our group, we study the possibility of using new ions for therapy, in collaboration with the group of Treatment Planning and Validation, and we investigate the molecular mechanisms involved in the formation of disseminating tumor cells and cancer stem cells.
- Radioprotection (Peto´s Paradox)
Although logic would suggest the contrary, cancer incidence is not related to body size (more cells) and species life span (more cell division). Large, long-living mammals, such as elephants, have no increased risk of developing cancer compared to small organisms. This lack of correlation is known in evolutionary biology as Peto’s Paradox.
In this group, and in collaboration with the group of Dr. Charlot Vandervoorde from the NRF iThemba LABS in South Africa, we investigate the molecular and cellular mechanisms responsible for this paradox in order to improve our knowledge of radiation protection.
- Development and characterization of innovative 3D in vitro models
Those models closely mimicking human cancers, in order to understand tumor onset, progression and response to radiation therapy.
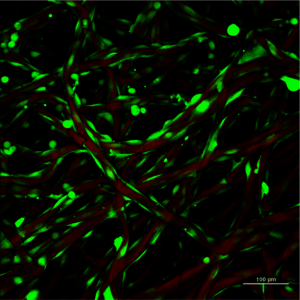
In this figure, an example of a biological phantom (3D in vitro model), made in collaboration with Dr. Bonani, (BIOTech, University of Trento, Italy). The CHO cells are growing on silk fiber immersed in an alginate gel (Alginic acid salt; alginates are widely used as thickeners and emulsifiers by the food and cosmetics industry).
- FLASH irradiation
Recent in-vivo investigations have shown that short pulses of electrons at very high dose rates (>40 Gy s−1) are less harmful to healthy tissues but just as efficient as conventional dose-rate radiation to inhibit tumor growth, suggesting that these so-called FLASH irradiations can substantially enhance the therapeutic window in radiotherapy. However, the mechanism remains unclear and a similar effect has not yet been proven for proton or ion beam irradiation. Whilst with cyclotrons these dose rates can be achieved for protons, it is more difficult with the synchrotrons used in heavy ion therapy.
3D printed modulators composed of fine and well-defined pyramid-shaped structures can be used to apply a highly conformal dose distribution of charged particles within a very short irradiation time (typ. 1-2 s; Fig. 1).
In the Clinical Radiobiology group, we are investigating, in collaboration with the group of Space Radiation Physics, the molecular mechanisms underlying this process.
Collaborations
• NIRS, Chiba, Japan
• HIT, Heidelberg, Germany
• DKFZ, Heidelberg, Germany
• iThemba, Cape Town, South Africa
• University of Bologna, Bologna, Italy
• University of Naples Parthenope, Naples, Italy
• University of Parma, Parma, Italy
• University of Rome, Rome, Italy
• Centro Fermi, Parma, Italy
• TIFPA, Trento, Italy
• INFN, Italy
Publications (2016 - 2019)
1) Bravatà V*, Tinganelli W*, Cammarata FP, Minafra L, Calvaruso M, Sokol O, Petringa G, Cirrone GAP, Scifoni E, Forte GI, Russo G. Hypoxia Transcriptomic Modifications Induced by Proton Irradiation in U87 Glioblastoma Multiforme Cell Line. Journal of Personal Medicine. 2021; 11(4):308.
2) Helm A, Tinganelli W, Simoniello P, Kurosawa F, Fournier C, Shimokawa T, Durante M. Reduction of Lung Metastases in a Mouse Osteosarcoma Model Treated With Carbon Ions and Immune Checkpoint Inhibitors. Int J Radiat Oncol Biol Phys. 2021; 109(2):594-602.
3) Puspitasari A, Cerri M, Takahashi A, Yoshida Y, Hanamura K, Tinganelli W. Hibernation as a Tool for Radiation Protection in Space Exploration. Life (Basel). 2021; 11(1):54.
4) Helm A, Tinganelli W, Fournier C, Simoniello P, Kurosawa F, Shimokawa T, Durante M. In Reply to Elmali et al.. Int J Radiat Oncol Biol Phys. 2021 Apr 1;109(5):1658-1659. doi: 10.1016/j.ijrobp.2020.12.0;
5) Tinganelli W, Durante M. Carbon Ion Radiobiology. Cancers (Basel). 2020; 12(10):3022.
6) Tinganelli W, Durante Marco. Tumor Hypoxia and Circulating Tumor Cells. Int J Mol Sci. 2020; 21(24):9592.
7) Kartini DA, Sokol O, Wiedemann J, Tinganelli W, Witt M, Camazzola G, Kraemer M, Talabnin C, Kobdaj C, Fuss MC. Validation of a pseudo-3D phantom for radiobiological treatment plan verifications. Physics in Medicine and Biology. doi: 10.1088/1361-6560/abb92d.
8) Tinganelli W, Hitrec T, Romani F, Simoniello P, Squarcio F, Stanzani A, Piscitiello E, Marchesano V, Luppi M, Sioli M, Helm A, Compagnone G, Morganti AG, Amici R, Negrini M, Zoccoli A, Durante M, Cerri M. Hibernation and Radioprotection: Gene Expression in the Liver and Testicle of Rats Irradiated under Syntetic Torpor. 2019; J Mol Sci. 20 (2).
9) Helm A, Ebner DK, Tinganelli W, Simoniello P, Bisio A, Marchesano V, Durante M, Yamada S, ShimokawaT. Combining Heavy-Ion Therapy with Immunotherapy: An Update on Recent Developments. International Journal of Particle Therapy. 2018; 5: 84-93.
10) Held KD, Story M, Grosshans D, Capala J, Blakely EA, Boerma M, Bortfeld T, Chang P, Combs SE, Durante M, Formenti S, Fornace AJ, Krishnan S, Limoli C, Paganetti H, Prise K, Stewart RD, Tinganelli W, Timmerman R, Vikram B, Willers H, Weil MM. Proceedings of the National Cancer Institute Workshop on Charged Particle Radiobiology. Int J Radiat Oncol Biol Phys 2018; 100:816-831.
11) Sokol O, Scifoni E, Tinganelli W, Kraft-Weyrather W, Wiedemann J, Maier A, Boscolo D, Friedrich T, Brons S, Durante M, Krämer M. Oxygen beams for therapy: advanced biological treatment planning and experimental verification. Phys Med Biol. 2017; 62(19): 7798-7813.
12) Tinganelli W*, Ebner DK*, Helm A, Bisio A, Simoniello P, Natale F, Yamada S, Kamada T, Shimokawa T, Durante M, the Abscopal Research Collaboration (ARC). Generating and grading the Abscopal Effect: Proposal for comprehensive evaluation of combination immunoradiotherapy in mouse models. Translational Cancer Research. 2017; (6).
13) Tinganelli W*, Ebner DK*, Helm A, Bisio A, Yamada S, Kamada T, Shimokawa T, Durante M. The Immunoregulatory Potential of Particle Radiation in Cancer Therapy. Front Immunol. 2017; 8: 99.
14) Cerri M, Tinganelli W, Negrini M, Helm A, Scifoni E, Tommasino F, Sioli M, Zoccoli A, Durante M. Hibernation for space travel: Impact on radioprotection. Life Sci Space Res (Amst). 2016; 11 1-9.
15) Scifoni E, Sokol O., Gruen R, Friedrich T, Scholz M, Tinganelli W, Brons S, Schuy C, Rovituso M, Durante M, Kraemer M. Helium and Oxygen beam models in TRiP98: implementation, treatment planning tests and experimental verification. Radiotherapy & Oncology. 2016; 118:S96.
16) Krämer M, Scifoni E, Schuy C, Rovituso M, Tinganelli W, Maier A, Kaderka R, Kraft-Weyrather W, Brons S, Tessonnier T, Parodi K, Durante M. Helium ions for radiotherapy? Physical and biological verifications of a novel treatment modality. Med Phys. 2016; 43 (4):1995.
17) Tinganelli W, Allen CP, Sharma N, Jie J, Sicard C, Natale F, King M 3rd, Keysar SB, Jimeno A, Furusawa Y, Okayasu R, Fujimori A, Durante M, Nickoloff JA. DNA Damage Response Proteins and Oxygen Modulate Prostaglandin E2 Growth Factor Release in Response to Low and High LET Ionizing Radiation. Front Oncol. 2015; 5: 260.
18) Tinganelli W, Durante M, Hirayama R, Kraemer M, Maier A, Kraft-Weyrather W, Furusawa Y, Friedrich T, Scifoni E. Kill-painting of Hypoxic tumorus in charged particle therapy. Sci Rep. 2015; 5: 17016.
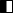

![Figure: a) Conformal dose distribution (12C, 400 MeV/u beam, sphere Ø= 5cm) produced by a 3D modulator, irradiation time ≈2 s [5]. b) Measured ‘Dose grid’ effect induced by a 3D modulator at different distances from the pa](/fileadmin/_processed_/9/e/csm_Conformal_dose_distribution_ae4a4f7045.png)