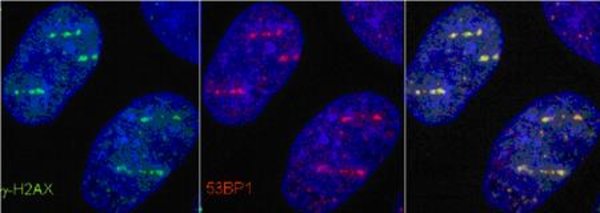
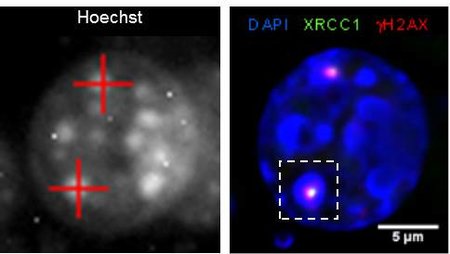
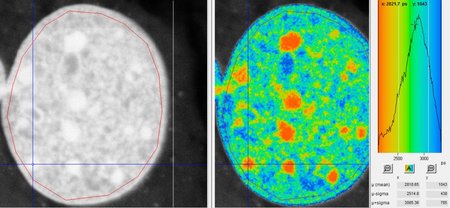
The main biological target of ionizing radiation action is the DNA molecule. The initial event leading to late radiation effects is indeed the DNA damage (primarily double-strand breaks) and its repair.
The Molecular Radiobiology & Imaging group studies the induction and repair of DNA in mammalian cells after exposure to heavy ions. The nature of the damage and its repair has indeed significant differences with respect to X-rays, due to the different physical dose deposition pattern. Moreover, the high lesion density induced by very heavy ions allows for accurate visualization and quantification of DNA repair protein recruitment.
Besides radiation quality, the influence of chromatin organization on repair is one of the major research topics. Especially densely packed, so called heterochromatic areas showed a retarded repair of double-strand breaks. Particle irradiation of heterochromatin, which can be studied exceptionally well in murine cells, induces a locally confined decompaction of the chromatin. This heterochromatic chromatin changes and subsequent repair of introduced lesions are topics of current research activities. Besides the establishment of sensitive microscopic techniques in this context, we address the question of the molecular processes involved and in how far this decompaction defines the success of repair.
The group exploits unique facilities such as the single particle heavy-ion microbeam (collaboration with Material Science Dept.) and live cell microscopy for the observation during and after irradiation with charged particles. For research on chromatin decompaction, the beamline microscope was equipped with a confocal scannerhead and fast pulsed lasers to measure fluorescence lifetime images (FLIM).
Main research topics
- Repair of complex DNA lesions
- Repair pathways (DSBs)
- Role of chromatin organization and remodelling
- Spatiotemporal Organization
- Role of epigenetics in repair Regulation
- Live-cell und innovative imaging
Collaborations
- Technical University of Darmstadt, Germany (Prof. M. Löbrich, Prof. C. Cardoso, Prof. G. Thiel)
- University of Duisburg-Essen, Germany (Prof. G. Iliakis)
- Saarland University, Homburg (Saar) (Prof. C. Rübe)
- University of Texas Southwestern, Dallas, USA (Prof. D. J. Chen)
- University of Queensland, Brisbane, Australia (Prof. M. Lavin)
- Natl. Inst. for Quantum and Radiological Science and Technology (QST), Takasaki, Japan, (Dr. Y. Kobayashi)
Funding
- BMBF 002NUK037A (VERCHROMT: Erkennung, Verarbeitung und biologische Konsequenzen von Chromatinschäden nach Teilchenbestrahlung)
- DFG GRK 1657 (Graduiertenkolleg: Molekulare und zelluläre Reaktionen auf ionisierende Strahlung / Graduate College: Molecular and cellular responses to ionizing radiation)
- HGS-HIRE (Helmholtz Graduate School for Hadron and Ion Research)
Group members
Research scientists:
Prof. Dr. Gisela Taucher-Scholz (Senior Scientist)
Dr. Nicole Averbeck (DNA Repair)
Dr. Katja Kratz (Post Doc)
Technicians:
Henrieke Förster
PhD students:
Dmitri Baulin
Amaya Krusch
Laura Schwan
Selected publications
Abdollahi E., Taucher-Scholz G., Durante M., Jakob B. (2015), Upgrading the GSI beamline microscope with a confocal fluorescence lifetime scanner to monitor charged particle induced chromatin decondensation in living cells. Nucl. Instr. and Meth. in Phys. Res. B, 365 B: 626-630
Averbeck N., Ringel O., Herrlitz M., Jakob B., Durante M., Taucher-Scholz G. (2014) DNA end resection is needed for the repair of complex lesions in G1-phase human cells. Cell Cycle.13(16):2509-2516. doi: 10.4161/15384101.2015.941743
Becker A., Durante M., Taucher-Scholz G., Jakob B. ATM alters the otherwise robust chromatin mobility at sites of DNA double-strand breaks (DSBs) in human cells. PLoS One. 2014 Mar 20;9(3):e92640. doi: 10.1371/journal.pone.0092640
Meyer, B., Voss, K.O., Tobias, F., Jakob, B., Durante, M. and Taucher-Scholz, G. (2013) Clustered DNA damage induces pan-nuclear H2AX phosphorylation mediated by ATM and DNA-PK. Nucleic Acids Res.41, 6109-6118
Jakob, B., Splinter, J., Conrad, S., Voss, K.O., Zink, D., Durante, M., Löbrich, M. and Taucher-Scholz, G. (2011) DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin. Nucleic Acids Res.39, 6489-6499
Jakob, B., Splinter, J., Durante, M. and Taucher-Scholz, G. (2009). Live cell microscopy analysis of radiation-induced DNA double-strand break motion. Proc Natl Acad Sci USA106, 3172- 3177